$93.99 Original price was: $93.99.$65.79Current price is: $65.79.
- Have the best deals
- Green products, guaranteed.
- Service with a smile, always online.
- Control the delivery date and try to deliver it in the fastest time

- HANDS-ON LEARNING OF STOICHIOMETRY & MOLE RATIOS || Grow beautiful silver crystals on copper wire to calculate the mole ratio of copper reacted to silver formed.
- DESIGNED BY SCIENCE EXPERTS || Labs are written by teachers and scientists and bench-tested for success, using materials designed to cut down on prep time and waste.
- COMPREHENSIVE INSTRUCTIONS || Separate Teacher’s Manual and Student Guide copymasters provide objectives, background info, step-by-step procedures, and data analysis questions.
- HIGH QUALITY CHEMICALS || Includes chemicals manufactured in the USA, with GHS-compliant labels and SDS for safety and storage information.
- ALIGNED TO THE NEXT GENERATION SCIENCE STANDARDS (NGSS) || Designed to meet specific objectives in the Next Generation Science Standards, which are listed in the Teacher’s Manual.
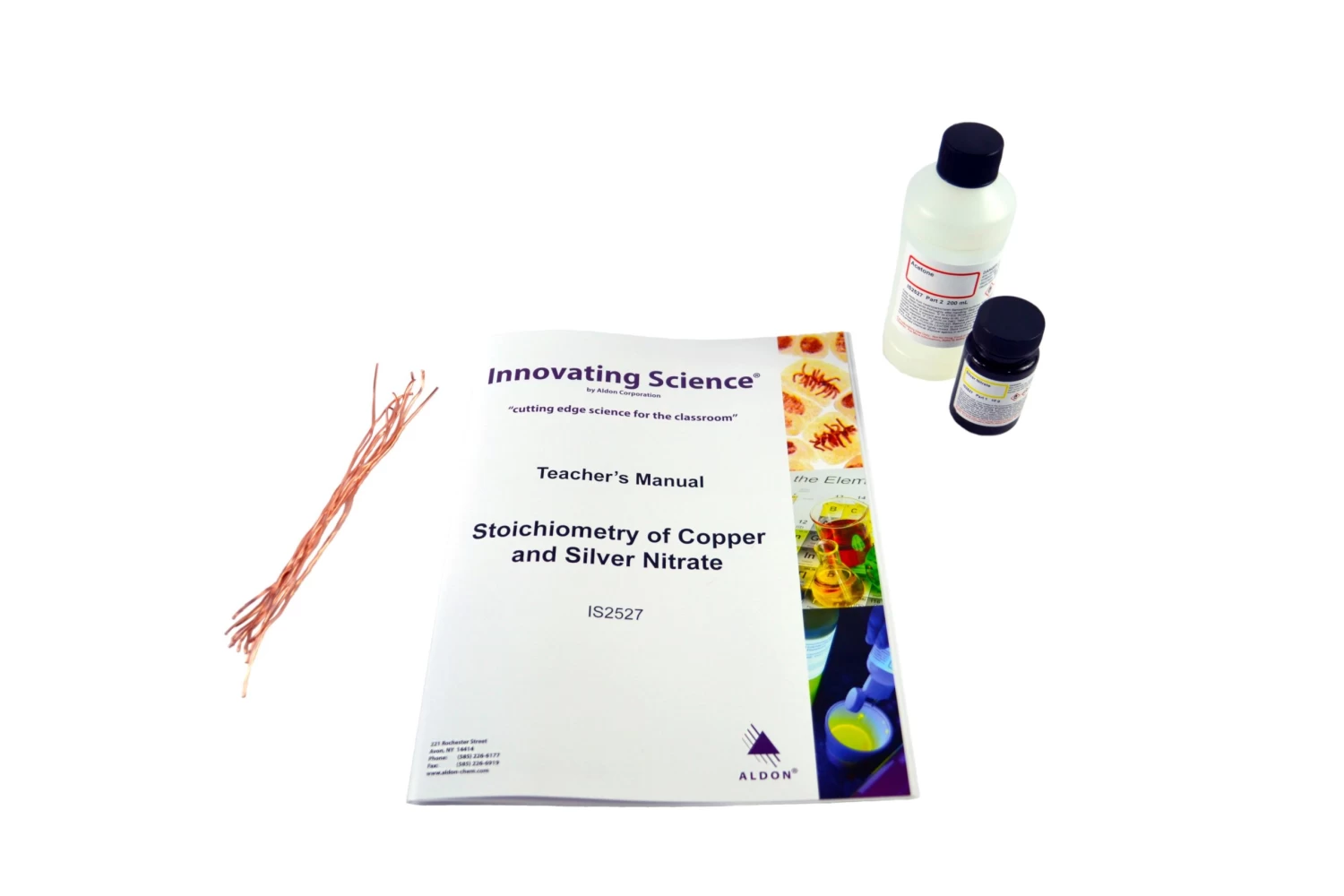
Introduce students to stoichiometry and mole ratios with the reaction of copper and silver nitrate! Students will watch as beautiful silver crystals are formed on the copper wire, then determine the moles of silver formed and moles of copper reacted to calculate the mole ratio. Using this data, they will write the balanced chemical equation for the reaction, and determine the limiting reactant and their percent yield. Kit contains enough materials for 15 groups of students. Teacher’s Manual and Student Study Guide copymasters are included.
Included in kit:
- 15 pcs Copper wire
- 10 g Silver nitrate
- 200 mL Acetone
Safety Data Sheet
Download Here
NGSS
Disciplinary Core Ideas:
PS1.A
PS1.B
Performance Expectations:
MS-PS1-2
HS-PS1-7
Cross Cutting Concepts:
Patterns
Energy and Matter
Engineering Practices:
Analyzing and Interpreting Data
Using Mathematics and Computational Thinking
Items Needed
Not included in kit:
- Large test tubes
- Test tube racks
- 250mL beaker
- Funnels
- Erlenmeyer flasks
- Stirring rods
- Weigh boats/paper
- Distilled or deionized water
Kit Specs
Regulated: Small Qty Exemption 49CFR173
Be the first to review “Kit, Stoichiometry Of Copper & Silver Nitrate – Materials For 15 Setups” Cancel reply
Related products
Chemistry
Activity Kits
Chemistry










Reviews
There are no reviews yet.